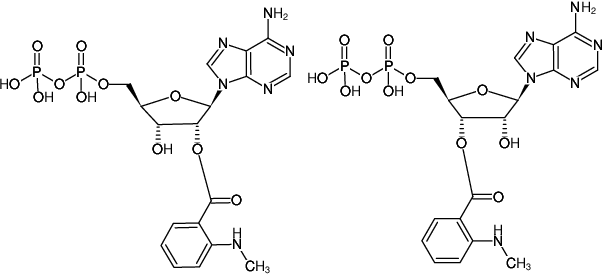
Mant-ADP
2'/3'-O-(N-Methyl-anthraniloyl)-adenosine-5'-diphosphate, Triethylammonium salt
| Catálogo Nº | Apresentação | Preço (R$) | Comprar |
|---|---|---|---|
| NU-201S | 150 μl (10 mM) | Sob demanda | Adicionar ao Carrinho |
| NU-201L | 5 x 150 μl (10 mM) | Sob demanda | Adicionar ao Carrinho |
For general laboratory use.
Envio: shipped on gel packs
Condições de armazenamento: store at -20 °C
Short term exposure (up to 1 week cumulative) to ambient temperature possible.
Validade: 12 months after date of delivery
Fórmula molecular: C18H22N6O11P2 (free acid)
Peso molecular: 560.35 g/mol (free acid)
CAS#: 151481-85-5
Pureza: ≥ 95 % (HPLC)
Forma: solution in water
Concentração: 10 mM - 11 mM
pH: 7.5 ±0.5
Propriedades espectroscópicas: λmax 255/355 nm, ε 23.3/5.8 L mmol-1 cm-1 (Tris-HCl pH 7.5), λexc 355 nm, λem 448 nm
Formulários:
MYOSIN-ATPase cycle[1]
SecA cycle[2]
Dissociation kinetic proteinkinase A[3]
FRET: kinesin[4], myosin[5, 1]
Conformational dynamic: DnaC-protein[6]
Specific Ligands:
Kinesin head domains[4]
Myosin[5]
Referências selecionadas:
[1] Robertson et al. (2005) Structural rearrangements in the active site of smooth muscle myosin. Biophysical J. 89:1882.
[2] Fak et al. (2004) Nucleotide exchange from the high-affinity ATP-binding site in SecA is the rate-limiting step in the ATPase cycle of the soluble enzyme and occurs through a specialized conformational state. Biochemistry 43:7307.
[3] Ni et al. (2000) Insights into nucleotide binding in protein kinase A using fluorescent adenosine derivatives. Protein Science 9:1818.
[4] Hackney et al. (2009) Half-site inhibition of dimeric kinesin head domains by monomeric tail domains. Biochemistry 48:3448.
[5] Sun et al. (2006) Dynamics of the upper 50-kDa domain of myosin V examined with fluorescence resonance energy transfer. J. Biol. Chem. 281:5711.
[6] Galletto et al. (2005) The nucleotide-binding site ot the Escherichia coli DnaC protein: Molecular topography of DnaC protein-nucleotide cofactor complex. Cell Biochem. and Biophys. 43:331.
Pinto et al. (2011) Structure-activity relationships for the interactions of 2'- and 3'- (O)- (N-methyl)anthraniloyl-substituted purine and pyrimidine nucleotides with mammalian adenylyl cyclases. Molecular Pharmacology 82 (4):358.
Chen et al. (2009) ADP but Not Pi Dissociation Contributes to Rate Limitation for Escherichia coli Rho*. J. Biol. Chem. 284 (49):33773.
Taha et al. (2009) Molecular Analysis of the Interaction of Anthrax Adenylyl Cyclase Toxin, Edema Factor, with 2' (3')-O- (N- (methyl)anthraniloyl)-Substituted Purine and Pyrimidine Nucleotides. Molecular Pharmacology 75 (3):693.
Del Toro Duany et al. (2008) The reverse gyrase helicase-like domain is a nucleotide-dependent switch that is attenuated by the topoisomerase domain. Nucleic Acids Research 36 (18):5882.
Kainov et al. (2008) Structural Basis of Mechanochemical Coupling in a Hexameric Molecular Motor. The Journal of biological chemistry 283 (6):3607.
Bujalowski et al. (2000) Kinetic mechanism of nucleotide cofactor binding to Escherichia coli replicative helicase DnaB protein. stopped-flow kinetic studies using fluorescent, ribose-, and base-modified nucleotide analogues. Biochemistry 39:2106.
Rice et al. (1999) A structural change in the kinesin motor protein that drives motility. Nature 402:778.
Cheng et al. (1998) Interaction of mant-adenosine nucleotides and magnesium with kinesin. Biochemistry 37:5288.
Bauer et al. (1997) X-ray crystal structure and solution fluorescence characterization of Mg-2' (3')-O- (N-methylanthraniloyl) nucleotides bound to the Dictyostelium discoideum myosin motor domain. J. Mol. Biol. 274:394.
Bujalowski et al. (1994) Structural characteristics of the nucleotide-binding site of Escherichia coli primary replicative helicase DnaB protein. Studies with ribose and base-modified fluorescent nucleotide analogs. Biochemistry 33:4682.
Moore et al. (1994) Kinetic mechanism of adenine nucleotide binding to and hydrolysis by the Escherichia coli Rep monomer. 1. Use of fluorescent nucleotide analogues. Biochemistry 33:14550.
Hiratsuka (1983) New ribose-modified fluorescent analogs of adenine and guanine nucleotides available as substrates for various enzymes. Biochim. Biophys. Acta 742:496.
