Dinucleotídeos Cíclicos
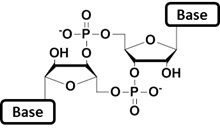
Cyclic dinucleotides (CDNs) span a highly pleiotropic class of bacterial second messenger molecules. Their cellular levels are subject to tight regulation by synthesis and breakdown[1]. By binding to their cognate effector proteins or riboswitch RNA, CDNs regulate numerous phenotypic features such as mobility[2], sessility[2], biofilm formation[3] and virulence[4]. In addition to a bacterial origin, recent findings suggested CDNs to be synthesized by metazoans metazoan cells, preparing the ground for an upcoming and exciting field of research[5].
Produtos
[1] Jenal et al. (2017) Cyclic di-GMP: second messenger extraordinaire. Nat. Rev. Microbiol. 15:271.
[2] Simm (2004) GGDEF and EAL domains inversely regulate cyclic di-GMP levels and transition from sessility to motility. Mol. Microbiol. 53:1123.
[3] Tischler et al. (2004) Cyclic diguanylate (c-di-GMP) regulates Vibrio cholerae biofilm formation. Mol. Microbiol. 53:857.
[4] Kulasakara et al. (2006) Analysis of Pseudomonas aeruginosa diguanylate cyclases and phosphodiesterases reveals a role for bis-(3′-5′)-cyclic-GMP in virulence. Proc. Natl. Acad. Sci. USA. 103:2839.
[5] Ablasser et al. (2013) cGAS produces a 2′‑5′‑linked cyclic dinucleotide second messenger that activates STING. Nature 498:380.
